Decomposition of Zinc Nitrate
The chemical formula of Zinc Nitrate is ZnNO32. The mixture of zinc nitrate.

The Action Of Heat On Zinc Ii Nitrate Laboratory Experiments On Salts Youtube
The controlled decomposition of zinc nitrate into zinc oxide is used in the generation of various zinc oxide-based structures like including nanowires.

. The decomposition reaction is NH4NO3 -heat- N2O 2H2O. Strong signals from NO NO 2 and O 2 as well as the absence of H 2 O indicate a. What is the equation for the decomposition reaction of zinc nitrate.
To balance Zn NO32 ZnO NO2 O2 youll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom. The gaseous fragments accompanying the decomposition of anhydrous zinc nitrate ie.
Aqueous solution of Zn NO 3 2 6H 2 O 30 mL 3 M pH 3 009 mol as Zn NO 3 2 6H 2 O was added in a single step to an equimolar amount of ZnO powder. It is a white. The reaction is given below.
The N-doped ZnO was prepared by heating a mixture of zinc nitrate hexahydrate ZnNO326 H2O and ammonium salt at 623 K for 1 h in air. Department of Energys Office of Scientific and Technical Information. On heating zinc nitrate Z n N O 3 2 it undergoes thermal decomposition to form zinc oxide nitrogen dioxide and oxygen.
This decomposition leaves behind no residue. Help the decomposition temperature of zinc nitrate iron nitrate. Application of in situ X-ray.
During the thermal decomposition of hydrated zinc nitrateV under non-isothermal conditions with any rate of heating even as low as 01 K min 1 no distinct stages. Zinc nitrate nine -water -alcoholic nitrate iron Mn NO3 2Now I need to set up these sets of data. Zn 2 HNO3 diluted Zn NO32 H2 4 Zn 10 HNO3 concentrated 4 Zn NO32 NH4NO3 3 H2O It undergoes thermal decomposition on.
Browse the articles related decomposition of zinc nitrate. Production of Zinc nitrate It is produced by dissolving zinc in nitric acid. Zn NO32 ZnO NO2 O2 The thermal decomposition of zinc nitrate The thermal decomposition of zinc nitrate 2Zn NO 3 2 2ZnO 4NO 2 O 2 Check the balance.
How many elements are there in a formula for nitrate. Zinc Nitrate ZnNO 3 2 is an inorganic chemical compound typically found in the form of a hexahydrate. The layered basic hydroxide Zn 5 OH 8 NO 3 2 2H 2 O can be thermally decomposed to ZnO via a series of intermediary compounds.
The reaction is concentration-dependent and forms ammonium nitrate. The reaction is given below. Read Or Download Gallery of chemical reactions - Zinc Nitrate Decomposition zinc reacting with silver nitrate stock image c030 7216 science zinc nitrate industrial chemicals.

How To Balance Zn No3 2 Zno No2 O2 Zinc Nitrate Decomposition Youtube
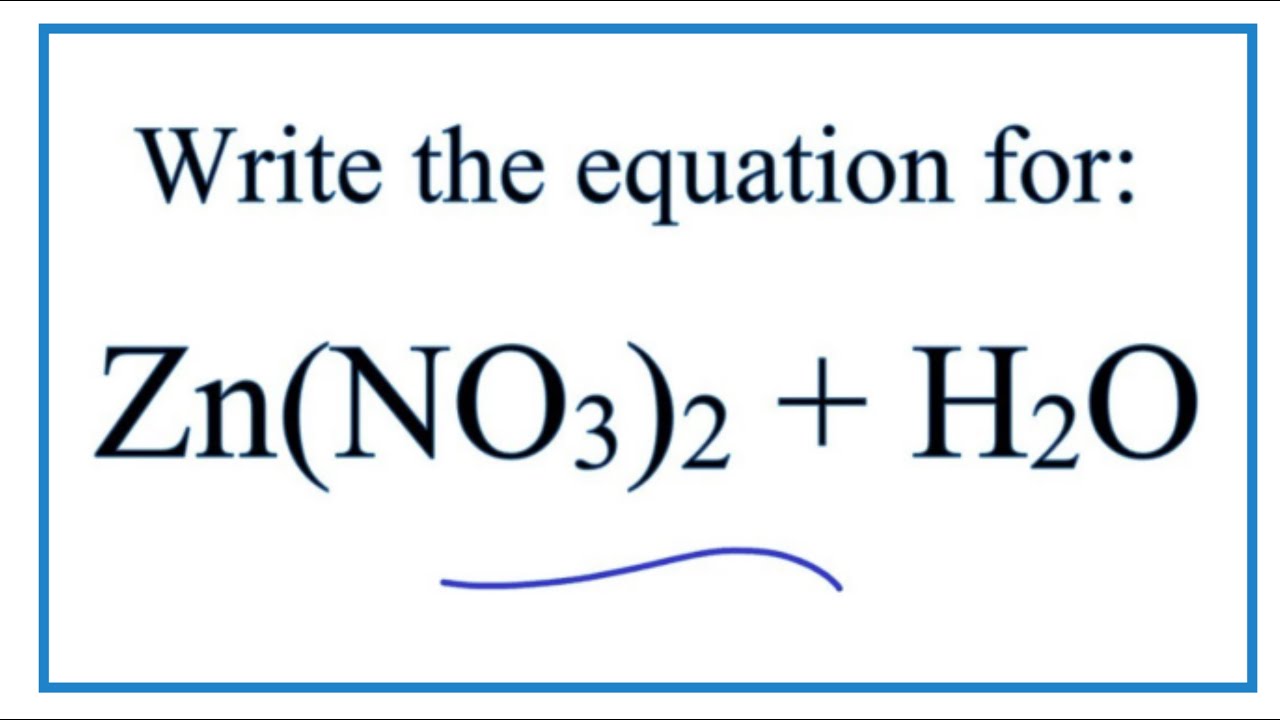
Equation For Zn No3 2 H2o Zinc Nitrate Water Youtube

How To Balance Zn No3 2 Zno No2 O2 Zinc Nitrate Decomposition Youtube

The Formula Of Zinc Nitrate Is
0 Response to "Decomposition of Zinc Nitrate"
Post a Comment